
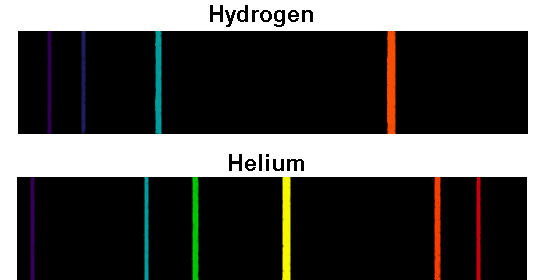
Scientist found that each element of periodic table has a unique set of spectral lines, as a signature of the element. This means that a heated gas emits light in just some specific wavelength. The spectrum of the heated gas was discrete lines or spectra lines. In 1850s, Anders Jonas Angstrom observed that the light emitted by a high heated gas did not have a continuum spectrum, like white light, when it passes through a prism. This was the beginning of the Spectrum Analyze Technique. In the 19 th century, scientist discovered that they could use the light emitted by heated or electrical discharged materials to analyze their properties. The table below shows the range of wavelength for each of seven colors of the rainbow. The result is that red light bends less sharply than violet as it passes through the prism, creating a spectrum of colors. The decomposition of the white light in different colors results from different wavelengths, as a consequence, they move at different speeds in the prism, with red light moving faster than violet. This means that the white light is formed by the combination all visible colors. In sequence of this experiment, Newton combined those colored beams in another prism which resulted in another white light beam. Newton classified this spectrum in a range of seven different colors (Red, Orange, Yellow, Green, Bleu, Indigo and Violet). this is sufficient resolution to resolve the profile of the Kr He-? line.In the 1670s, Isaac Newton, during optical experiments observed that a beam of white light was decomposed in a continuum spectrum of all visible colors, like a rainbow, when it pass through a prism. In a recently-published paper, diagnostic methods for laser implosions were proposed, using Krypton K-shell x-ray lines, particularly the He-? line at 15.43 keV (or 0.8 2000 - 3000. The intensities for the lines from each ion found in the data-base search can be scaled by a multiplicative constant to better simulate the observed spectrum. Options for manipulating the experimental spectra's background intensity and wavelength scale are also available to the user. It is also possible to bring in data from another source, such as an experimental spectra, for display along with the lines from the data-base search. Several options are available to control the nature of these graphs. A plotting capability is provided to graphically display the lines resulting from the search. The line information found in the search is stored in a local file for later reference.
ARGON ATOMIC EMISSION SPECTRUM CODE
This code allows the user to search the data base for a user-specified wavelength region, with this search either limited to atoms or ions of the user's choice for all atoms and ions contained in the data more » base. The data base was the basis for the Kelly and Palumbo critical review of well-resolved lines below 2000 Angstroms, includes lines below 3500 Angstroms for atoms and ions of hydrogen through krypton, and was obtained from R.L. An interactive computer code has been written to search a data base containing information useful for identifying lines in experimentally-observed spectra or for designing experiments.


 0 kommentar(er)
0 kommentar(er)
